Introduction
Analysis of enrichment of UO2 is important to verify the information declared by the license-holders. The redundancy methods are required to guarantee the analysis result during the verification. Alpha spectrometry and Thermal Ionization Mass Spectrometry (TIMS) are one of methods for verification. Korea Institute of Nuclear Nonproliferation And Control (KINAC), the technical support organization to implement the safeguards, had established alpha spectrometry first.
Alpha spectrometry could analyze the sample which is suspected that includes a variety of isotopes, such as 238U and 238Pu [1]. In addition, it takes less time and cost of the pretreatment and analysis than TIMS. KINAC used to analyze the UO2 materials with alpha spectrometry to verify the declared information, also consign the TIMS to Korea Basic Science Institute (KBSI), one of the KINAC's network laboratories as a redundancy method [2].
This article evaluated the similarity of the results with two methods, and derived correlation equation. This equation could be adapted to make a criterion for verification of declared information. In addition, it is expected that the predictability of uranium enrichment with alpha spectrometry would be increased in a timely manner, before establishing TIMS.
Materials and Methods
One mg of UO2 powder samples from each 34 different uranium pellets put into 8 mL of 8M HNO3 solution. These 34 uranium pellets, which have the wide range of uranium enrichment were used for analyzing the enrichment with alpha spectrometry. The samples are not certified, since those were sampled from the license-holders. It makes the uranium enrichment value analyzed by TIMS as a comparison parameter.
The uranium tracer is not added to analyze uranium enrichment to calculate the recovery factor, since the uranium isotopes have same chemical behavior during pretreatment of the sample. The purification process with the resins was omitted because uranium pellet has only uranium oxide and its daughter nuclides. Also, the energies emitted from the alpha particles of daughter nuclides are not similar to those from the uranium isotopes.
The solution was dissolved using Microwave dissolver (START D, Milestone Inc., Sorisole, Italy) at 400 W, 110°C for 2 hours. The 100 μg solution loaded into the 15 mL Teflon vial was evaporated to dry. Then, the NH4OH and dilute H2SO4 10% (vol/vol) was added into that to adjust the pH about 1.8, at which the electrodeposition is performed best [3]. The solution was transferred to Teflon cell for electrodeposition. It was carried out for two hours, with a current of 1 A. Uranium shall be plated on the polished stainless-steel disk used as a cathode. One mL of NH4OH was added, one minute before the electrodeposition was completed. The disc was heated by the torch after the electrodeposition. Four pellets from the 34 pellets were chosen to make ten extra disc-samples to check the reproducibility, when comparing the effect of correction.
Alpha spectrometer (Canberra Industries, Inc., Meriden, CT) is composed with the multichannel pulse-height analyzer, the detector bias supplier, and the Passivated Implanted Planar Silicon detector (size: 450 mm2, resolution: 18 keV) in a vacuum chamber. 66.66 Pa for pressure inside the chamber was maintained during the measurement. The disks were measured for 80,000 seconds in a live time with the GENIE-2000. The energy and the efficiency were calibrated with the standard reference source (Eckert & Ziegler SRS 102430, thick stainless-steel disk) including the 238U, 234U, 239Pu, and 241Am.
The enrichment using alpha spectrometry was calculated as following equation (1). Yxs for the yields of uranium are shown in Table 1.
where:
Results and Discussion
Table 2 shows uranium enrichment values evaluated with TIMS and alpha spectrometry. It shows two results are not perfectly matched, although they should be similar to each other if the samples are analyzed well. The enrichment values of alpha spectrometry were estimated to be 9% to 55% greater than those of TIMS, and it was 17% higher on average. However, it has a tendency for the enrichment with alpha spectrometry to be higher than that of TIMS.
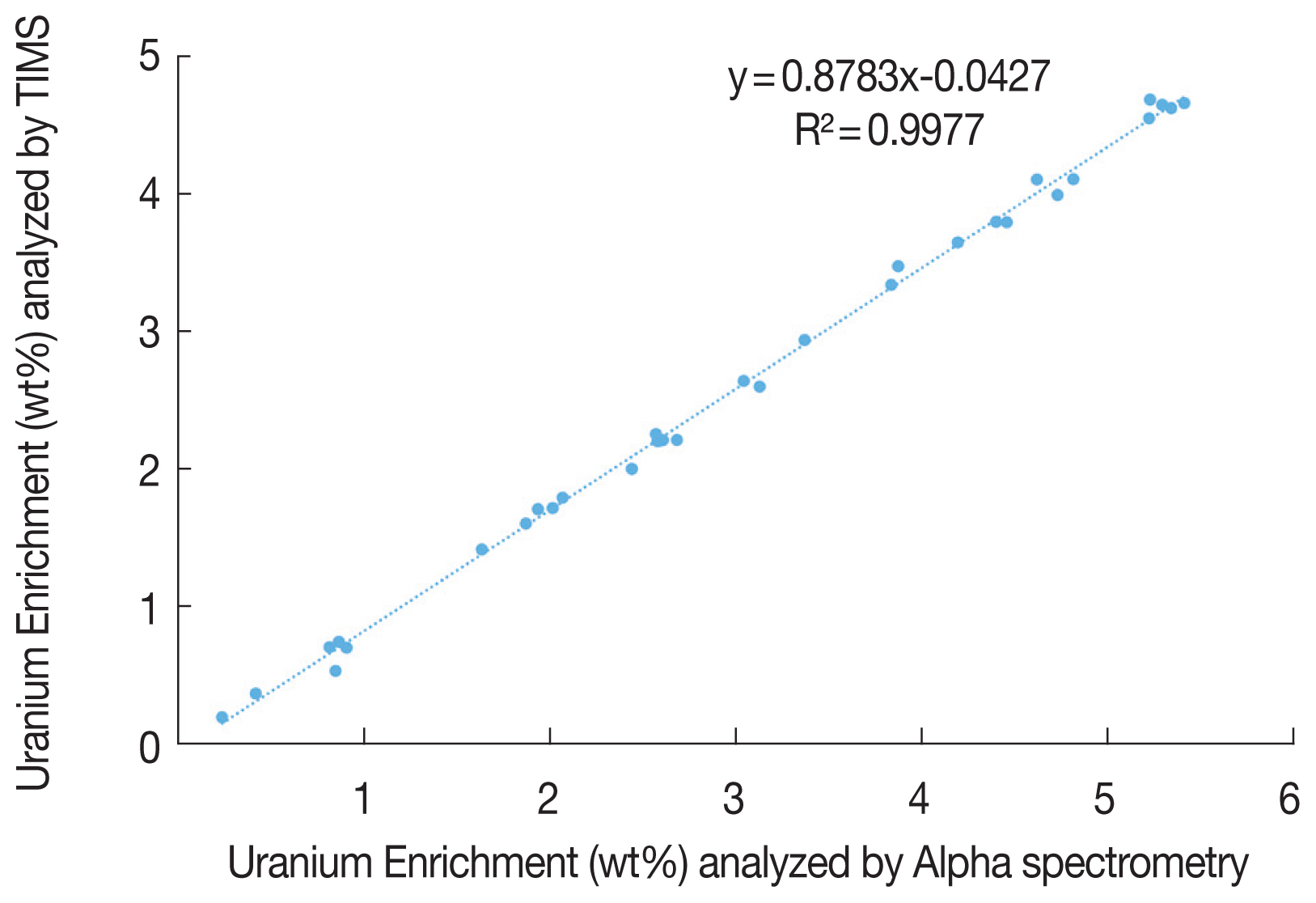
The linear fitting line in the Figure 1 shows the correlation. The regression equation (y=0.8783x-0.0427) was derived. The axis (x) and (y) stand for the uranium enrichment with alpha spectrometry and TIMS, respectively. The coefficient of determination, R2, is 0.9977. It means this equation is appropriate to predict the enrichment values by TIMS with that of alpha spectrometry.
KINAC had repeated the experiment to check the prediction possibility for enrichment by TIMS with that by alpha spectrometry. There are concentrated areas around 0.7, 1.8, 3.5, 4.1 wt% with TIMS, in this Figure 1. These four pellets are chosen based on this, ten samples for each pellet were made and detected repeatedly. Table 3 shows the mean enrichment with the standard deviation by alpha spectrometry. It shows the deviation is affected by the peak counts, but not enrichment.
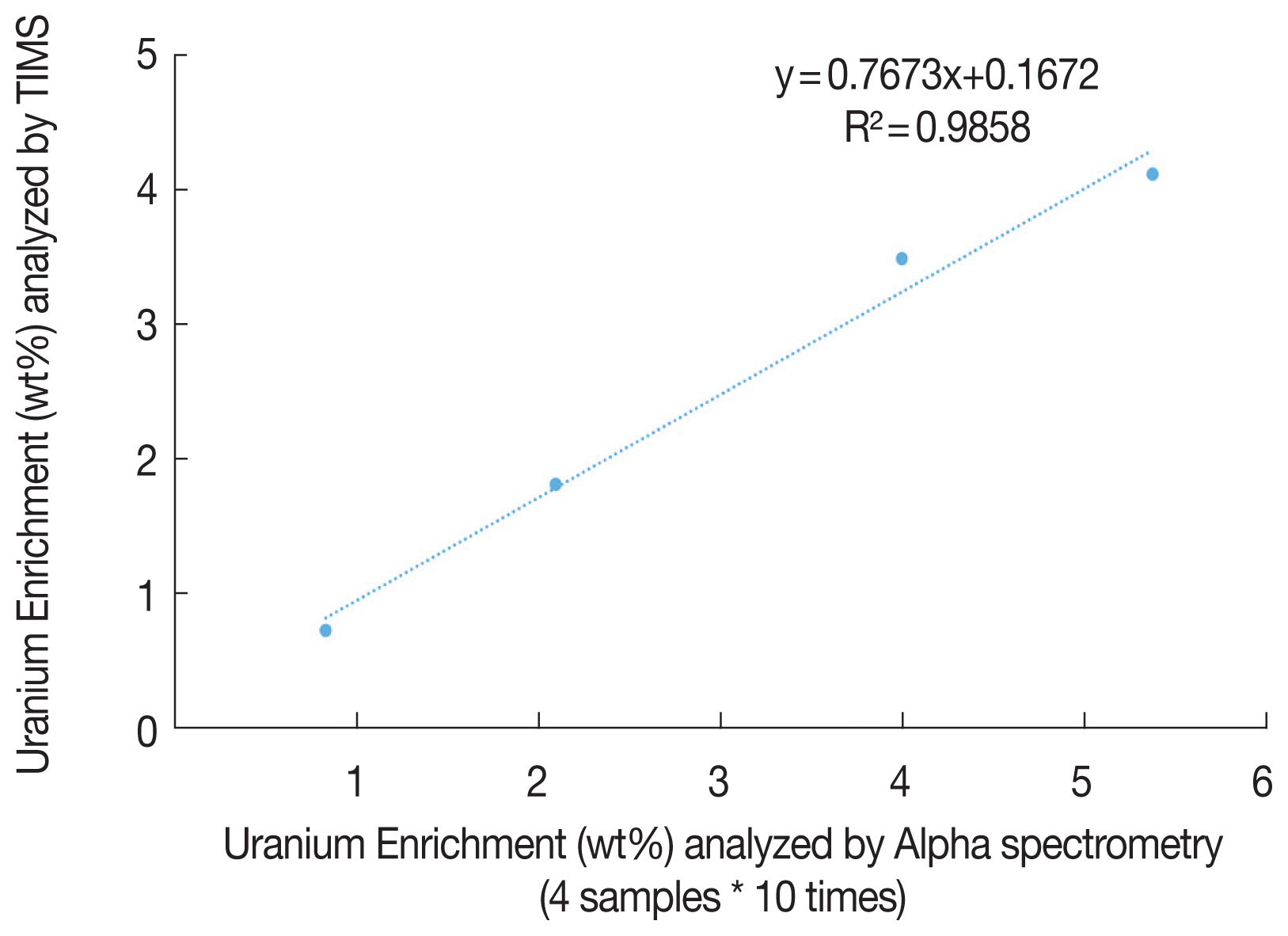
Figure 2 shows the linear fitting line derived from the enrichment of 4 pellets by TIMS and that of four pellets with ten times by alpha spectrometry. The equation was (y=0.7673x+ 0.1672). The comparison ratio (0.7673 with 40 samples) is decreased than that with 34 pellets. The discrepancy between the lowest and highest enrichment seems to be one of the reason for the comparison ratio decrease.
It also shows the R2 is 0.9858, which is less than 0.9977 evaluated with 34 pellets. It indicates a decrease in the prediction probability with enrichment by alpha spectrometry to infer that by TIMS, although the number of replicated samples had increased.
Conclusion
The study shows there are the tendency of analyzed enrichment by each equipment. It shows uranium enrichment with alpha spectrometry in KINAC evaluated 17% higher than that with TIMS on average.
The regression equations were also derived in case the similarity between the two results with two methods is lower than predicted. Two experiments were designed to compare the effect of number of samples. The R2 was 0.9977 with 34 pellets. It shows the equation is appropriate to predict the enrichment values by TIMS with that of alpha spectrometry. The R2 was 0.9858 with 40 samples. The R2 decreased while the number of samples increased. The discrepancy between the lowest and highest enrichment seems to be one of the reason for it. KINAC expects the first equation with 34 samples is useful to predict the result with TIMS, the redundancy method, based on the alpha spectrometry. The extra samples are necessary to collect if the enrichment value analyzed by TIMS is lower than the value predicted with the equation.
This article expects these regression methods could be helpful to compare the results from the different equipment or different operators. It could also be useful to compare the proficiency of the operators. Further study would be followed related to the impact of the peak counts for each uranium isotopes, sample amount and number of experiments when TIMS established in KINAC by the end of 2018.