Radioactive Concentrations in Chemical Fertilizers
Article information
Abstract
Background
The aim of the present study was to determine radioactive concentrations in fertilizers known to contain essential nutrients. Results of this study could be used as basic data to monitor the impact of chemical fertilizers on the environment and public health. Nitrogen fertilizers, calcium fertilizers, sulfur fertilizers, phosphate acid fertilizers, and potassium chloride fertilizers were used in this study.
Materials and Methods
Five chemical fertilizers were pulverized, placed in polyethylene containers, and weighed. The time to measure each specimen was set to be 3,600 seconds for a scintillator-based gamma-ray spectroscopy system. Concentration of gamma radionuclide was analyzed based on obtained spectra. At the end of the measurement, the spectrum file was stored and used to calculate radioactive concentrations using a gamma-ray spectrometer software.
Results and Discussion
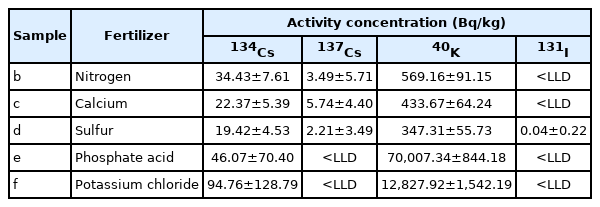
In the nitrogen fertilizer, 3.49±5.71 Bq/kg of 137Cs, 34.43±7.61 Bq/kg of 134Cs, and 569.16±91.15 of 40K were detected whereas 131I was not detected. In the calcium fertilizer, 5.74±4.40 Bq/kg of 137Cs (the highest concentration among all fertilizers), 22.37± 5.39 Bq/kg of 134Cs, and 433.67±64.24 Bq/kg of 40K were detected whereas 131I was not detected. In the sulfur fertilizer, 347.31±55.73 Bq/kg of 40K, 19.42±4.53 Bq/kg of 134Cs, 2.21± 3.49 of 137Cs, and 0.04±0.22 Bq/Kg of 131I were detected. In the phosphoric acid fertilizer, 70,007.34±844.18 Bq/kg of 40K (the highest concentration among all fertilizers) and 46.07± 70.40 Bq/kg of 134Cs were detected whereas neither 137Cs nor 131I was detected. In the potassium chloride fertilizer, 12,827.92±1542.19 Bq/kg of 40K was and 94.76±128.79 Bq/kg of 134Cs were detected whereas neither 137Cs nor 131I was detected. The present study examined inorganic fertilizers produced by a single manufacturer. There might be different results according to the country and area from which fertilizers are imported. Further studies about inorganic fertilizers in more detail are needed to create measures to reduce 40K.
Conclusion
Measures are needed to reduce radiation exposure to 40K contained in fertilizers including phosphoric acid and potassium chloride fertilizers.
Introduction
Ionizing radiation was discovered by German physicist Wilhelm Conrad Rontgen in 1895. Since then, radiation has been used in various industries such as manufacturing, agriculture, health, and high technologies [1]. Accordingly, more and more industry sectors are using radiation after understanding its characteristics and energy properties. Radiation technologies are being more widely used in the area of research and development for daily necessities, including the health industry, advanced parts manufacturing, biotechnology, and new variety development [2]. With the use of radiation, the quality of life has been improved along with development of industries and economies [3]. On average, our radiation exposure due to all natural sources amounts to about 2.4 mSv a year [4]. It is considered that natural radiation has a minimal effect on the human body. However, the International Commission on Radiological Protection has warned that risks of radiation exposure are likely to increase proportionately with higher exposure to radiation even in small doses [5]. It has also been reported that plants growing in a soil rich in natural radionuclides coming from uranium or thorium can play a part in internal exposure to radioactive elements that we take into our bodies [6]. Phosphate rock, a pre-concentrated phosphate ore, is the primary raw material for the production of mineral phosphate fertilizer. It contains 0.02% of uranium. In its by-product, phospho-gypsum, radioactive elements including radium exist. Caesium-137 is one of the radionuclides from various human activities, such as the spread of nuclear materials, for some reasons. It can cause food contamination and internal exposure to radiation [7]. After the Fukushima Daiichi accident in 2011, massive efforts have been put together to regulate and decrease 137Cs in food items in Japan [8–10]. There are pertinent risks of carcinogenesis related to internal radiation doses due to food contaminated by radioactive materials including 134Cs, 137Cs, and 131I according to Korea National Health and Nutrition Examination Survey. Nuclear-fuel reprocessing plants, nuclear wastes, and geological repositories are main sources of radioactive iodine in the environment [11, 12]. Conventionally, radiation exposures to uranium, radium, thorium, and potassium are well accounted for due to their roles in radioactivity from the environment. However, after recent accidents such as the Fukushima Daiichi accident in 2011, it is now of dire importance to measure radiation levels in Southeast Asia generally and Korea specifically due to its very close proximity to Fukushima, Japan [13, 14]. Iodine-131 is a very critical radioisotope which is closely associated with nuclear fission reactions. It causes environmental hazards in case of nuclear bomb attacks/mishaps and nuclear power plant accidents [15]. In case of a disaster or incident involving the spread of nuclear waste or a nuclear attack, the resultant radioactivity can spread up to 300 miles. The most extravagant health hazard from exposure to 131I is an enhanced risk of cancer induced due to radiation. It can also result in deformities, non-cancerous growths, and thyroiditis [16]. Accidents such as nuclear-reactor incidents at Chernobyl in 1986, the incident of Three Mile Island in 1979, and the most recent Fukushima nuclear reactor accident in 2011 provide clear evidence that it is urgent to understand health hazards posed by the presence of iodine in the atmosphere [17]. After the Fukushima incident, Korean authorities are taking threats of food-based radiation very seriously. The Ministry of Food and Drug Safety in Korea has conducted surveys to measure radioactivity levels of 134Cs, 137Cs, and 131I through the Korea National Health and Nutrition Examination Survey [12]. Geographically, Korea is the closest country to Japan. Therefore, there is a dire need to confirm the absence or presence of 131I in the environment and soil. Although the quantity of radiation by each radioactive element individually can be below the standard hazardous level or the maximum tolerance of 370 Bq/kg for any food, fertilizers with multiple radioactive elements used for farming might deliver radiation to agricultural products and consequently contaminate water reserves such as streams and groundwater after mixing with rain or irrigation water [6, 18–22]. Although there are a number of studies available about radioactivity due to 238U, 232Th, and 40K present in chemical fertilizers from countries such as Serbia, Bangladesh, India, and Iraq [23–26], studies that measure the radioactivity of agricultural fertilizers in Korea and the rest of the world are lacking. Studies generally involve 137Cs and 130I specifically. A variety of reports have analyzed radioactivity levels in water, air, and soil [27–29]. Hence, this study will serve as a foundation to better monitor the impact of radioactive levels in chemical fertilizers on public health and environment and to ensure that radiation levels in chemical fertilizers that contain essential nutrients for plant growth and development are safe for human health and the environment.
Materials and Methods
1. Experimental Materials
A total of five fertilizers, nitrogen fertilizers, calcium fertilizers, sulfur fertilizers, phosphate acid fertilizers, and potassium chloride fertilizers, were used as experimental fertilizers. They are the most widely used fertilizers in Korea. Distilled water was used as a control (Fig. 1). Nitrogen fertilizers contained 46 % of nitrogen. Calcium fertilizers had 60% of calcium. Sulfur fertilizers had 24% of sulfur. Phosphate acid fertilizers had 50% of soluble phosphate acid, 32% of soluble chloride, 0.05% of soluble copper, and 0.05% of soluble zinc. Potassium chloride fertilizers had 60% of potassium. Fertilizers are named with the highest content of the main ingredient.
2. Methods
1) Sample preparation
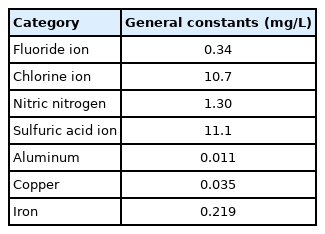
Five specimens (nitrogen fertilizer, calcium fertilizer, sulfur fertilizer, phosphate acid fertilizer, and potassium chloride fertilizer) and distilled water as control were used (Table 1). Each of these five specimens was pulverized and placed in a 500 mL of Marinelli vessel (Fig. 2). The density of the distilled water ranged from 0.998 g/cm3 at 20 °C to 0.996 g/cm3 at 30 °C. Compositions of distilled water are shown in Table 2.

Chemical fertilizers in scintillator. An empty container as a background (A), nitrogen (B), calcium (C), sulfur (D), phosphate acid (E), and potassium chloride (F) fertilizers are shown.
Each container was weighed on a scale. This time, the container weight was not considered. The net weight was marked on each container and numbered. The distilled water and each specimen were placed on a thallium-activated sodium iodide, NaI(Tl), scintillator-based gamma-ray spectroscopy system (gamma-ray spectrometer RUG 91-2; LINEV Systems, Minsk, Belarus) and measured. External radiation was blocked with a shield consisting of 48% lead, 45% tungsten, 5% iron, and 2% nickel with a thickness of 10 cm. Characteristics of the NaI(Tl) detector are provided in Table 3. The NaI(Tl) detector can respond to a gamma ray by producing a small flash of light or a scintillation. The scintillation occurs when scintillator electrons excited by energy of photon return to their ground state. The detector crystal is mounted on a photomultiplier tube which converts scintillation into an electrical pulse. This is taken from the anode of the photomultiplier. It is a negative pulse. The NaI(Tl) crystal is protected from the moisture in the air by encasing it in aluminum, which also serves as a convenient mounting for the entire crystal/photomultiplier unit.
2) Spectrometric analysis
RUG 91-2 gamma-ray spectrometer (radiometer) was used to analyze the amplitude of photon distribution of pulses generated from the scintillation detector upon detection of gamma-ray of the sample. Amplitude distribution of pulses was then analyzed and radionuclide activity was calculated. In order to enhance the registration of photon impulses, the sample was placed into a 0.5-L Marinelli vessel installed in a lead shield which could protect the sample from the influence of an external background radiation. Calibration and check took only a few minutes. Photo peaks of gamma-ray at 1461 keV, 622 keV, 796 keV, and 365 keV were used for measuring 40K, 137Cs, 134Cs, and 131I, respectively.
Distilled water as control and each fertilizer specimen were counted for 3,600 seconds. Gamma radionuclide concentration was then analyzed to obtain a spectrum file. At the end of each measurement, the spectrum file which had been stored was analyzed using a gamma-ray spectrometer software. Based on the analysis results, radiation level of each specimen was then determined.
Results and Disucssion
The nitrogen fertilizer had 3.49±5.71 Bq/kg of 137Cs, which was the second highest concentration, 34.43±7.61 Bq/kg of 134Cs, and 569.16±91.15 Bq/kg of 40K, whereas 131I was not detected. The calcium fertilizer had the highest concentration of 137Cs at 5.74±4.40 Bq/kg. It also had 22.37±5.39 Bq/kg of 134Cs and 433.67±64.24 Bq/kg of 40K. However, 131I was not detected. The sulfur fertilizer had 347.31±55.73 Bq/kg of 40K, 19.42±4.53 Bq/kg of 134Cs, 2.21±3.49 Bq/kg of 137Cs, and 0.04±0.22 Bq/kg for 131I. The phosphoric acid fertilizer had the highest concentration of 40K at 70,007.34±844.18 Bq/kg and 46.07±70.40 Bq/kg of 134Cs, whereas neither 137Cs nor 131I was detected. The potassium chloride fertilizer had 12,827.92± 1,542.19 Bq/kg of 40K and 94.76±128.79 Bq/kg of 134Cs. Neither 137Cs nor 131I was detected (Table 4). Fig. 3 shows radioactive concentrations and spectra of the five fertilizers and distilled water measured for 3,600 seconds.

Radioactive concentration spectrum of each fertilizer measured for 3,600 seconds. Spectra of background (A), nitrogen (B), calcium (C), sulfur (D), phosphate acid (E), and potassium chloride (F) are shown.
Currently, radioactive substances manufactured or processed using radiation technologies are being accumulated worldwide. They are mostly radioactive wastes produced from nuclear power plants, fallouts from nuclear tests, and radioisotopes used in the health industry [30]. Due to some nuclear disasters such as the Chernobyl nuclear disaster in 1986 and the Russia nuclear disaster in 1993, people are concerned about impacts of radiation on human health [31]. As many people are exposed to radionuclides from natural radiation sources in the soil due to such accidents, it is important to minimize radiation impacts. Zmazek et al. [32] have reported that areas rich in granite are more likely to have uranium and radon than non-granite areas. Granites include 2–12 parts per million (ppm) of uranium and 8–33 ppm thorium [33]. Every year, the Korea Institute of Nuclear Safety measures radiation in subsoil and topsoil for 14 areas and publishes analysis results due to concern about radiation impacts. Thus, the objective of this study was to measure radiation levels of inorganic fertilizers widely used in agriculture. As a result, 40K showed the highest levels in fertilizers containing phosphate acid and potassium chloride. A phosphate acid fertilizer’s base material is phosphate rock, a natural radiation source which contains 235U, 238U, and 232Th. Phosphate rock exporters to Korea are Morocco, China, Israel, and Togo. It was found that rocks they exported exceeded the radiation standard (1,000 Bq/kg) [34] in terms of 235U and 238U. As potassium chloride is added in order to improve hygroscopic capacity in the process of manufacturing a phosphate acid fertilizer, the level of 40K increases accordingly [35]. A potassium chloride fertilizer is manufactured with leucite, alunite, and sericite known to be rich in potassium. It has been reported that ores rich in potassium imported by Korea from countries such as Canada, Belarus, and Russia have high 40K levels, exceeding 1,000 Bq/kg. Almost all foods have natural radioactivity due to radioactive isotopes. For example, 40K is present at 40–50 Bq/kg in cows’ milk, 400–500 Bq/kg in milk powder, 600–800 Bq/kg in concentrated fruit juice, and over 1000 Bq/kg in instant coffee [22]. Natural radioactivity is primarily due to primordial radionuclides 40K, 232Th, 238U, 235U, and so on present in different quantities depending on soils of different regions in the world [36]. According to the International Atomic Energy Agency, a permissible limit for food is 370 Bq/kg of 40K [35]. However, Rajacic et al. [25] have demonstrated that the concentration of 40K in fertilizer is 4,860 Bq/kg [25]. Radiation due to 40K, a radionuclide, is rapidly absorbed into the body, causing internal exposure to radioactive elements that we take into our bodies through food and water [37]. In the environment, 40K is redistributed through agriculture by application of fertilizers. Thus, its concentration should be measured to distinguish safe utilization levels of fertilizers rich in 40K [19, 25, 38]. From production to utilization in agriculture, fertilizers might cause exposure to gamma radiation (external exposure) [39–41]. Many biomedical hazards are associated with exposure to radiation for a few weeks or months to years, which might cause radiation-induced liver disease [42–45]. Exposure to a significant level of radiation released from fertilizers can potentially cause cancers. Thus, a deeper understanding of fertilizers-based radiation is required [46, 47]. In particular, 40K is a constant and uniform source of exposure to radiation for humans [48]. The present study examined inorganic fertilizers produced by a single manufacturer. Results might be different depending on the country and areas from which fertilizers are imported. Further studies about inorganic fertilizers in more details are needed to create measures to reduce 40K.
Conclusion
As a result of this study, 40K had the highest levels in five fertilizers. Thus, there is a need to take measures to reduce radiation exposure from 40K contained in fertilizers including phosphoric acid and potassium chloride. This study can help us understand radioactive concentrations in five inorganic fertilizers used in Korea. In the future, it is necessary to set up measures to cut radiation impacts from radionuclides.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Author Contribution
Conceptualization: Kim GH. Project administration: Cho JH. Writing - original draft: Kim GH. Writing - review and editing: Cho JH. Approval of final manuscript: all authors.