AbstractBackgroundAptamers are currently being used in various fields including medical treatments due to their characteristics of selectively binding to specific molecules. Due to their special characteristics, the aptamers are expected to be used to remove radionuclides from a large amount of liquid radioactive waste generated during the decommissioning of nuclear power plants. The radiological effects on the aptamers should be evaluated to ensure their integrity for the application of a radionuclide removal technique.
Materials and MethodsIn this study, Monte Carlo N-Particle transport code version 6 (MCNP6) and Monte Carlo damage simulation (MCDS) codes were employed to evaluate the radiological effects on the aptamers. MCNP6 was used to evaluate the secondary electron spectrum and the absorbed dose in a medium. MCDS was used to calculate the DNA damage by using the secondary electron spectrum and the absorbed dose. Binding experiments were conducted to indirectly verify the results derived by MCNP6 and MCDS calculations.
Results and DiscussionDamage yields of about 5.00×10−4 were calculated for 100 bp aptamer due to the radiation dose of 1 Gy. In experiments with radioactive materials, the results that the removal rate of the radioactive 60Co by the aptamer is the same with the non-radioactive 59Co prove the accuracy of the previous DNA damage calculation.
IntroductionIn 1990, Ellington and Szostak [1] discovered RNA molecules that bind specific ligands and termed these molecules ‘Aptamer,’ from the Latin ‘aptus,’ meaning to fit. The aptamers are single-stranded DNA or RNA that, through intramolecular interactions, fold into unique tertiary conformations capable of binding to a target with a high affinity and specificity [2]. According to the high affinity and specificity, the aptamers can bind to the targets, such as metal ions, chemical compounds, proteins, cells, and whole micro-organisms [3].
Due to these target binding characteristics, the aptamers have been studied and utilized in a range of fields. In particular, the aptamers are similar to antibodies but have advantages such as high chemical stability and productivity compared to the antibodies, so they have been studied as alternatives to the antibodies [4, 5]. For example, by using the aptamers that can bind to a specific cell such as a cancer cell, the aptamers are used as a drug delivery agent [6].
Also, the aptamers can be used to develop aptamer-based new drugs, and in 2005, Pfizer developed an neovascular age-related macular degeneration treatment called ‘Macugen (pegaptanib sodium; Pfizer, New York, NY, USA),’ which was first marketed with approval of the U.S. Food and Drug Administration [7]. In addition, many studies have been conducted to apply the aptamers to diagnostics [8–10], aptamer-based biosensors [11, 12], and imaging systems [13].
Even though the aptamers are applied in so many fields as above described, there is no noticeable movement in the nuclear power field to utilize the target-binding characteristics of the aptamers. Therefore, this study suggested that the aptamers could be applied to the treatment of liquid radioactive waste (hereinafter referred to as “liquid waste”) generated in nuclear power plants by developing the aptamers that can bind to specific radioactive materials.
During the reactor operation, a large amount of the liquid waste is generated mainly by the coolant used to transfer thermal energy produced in nuclear fission [14]. Currently, in the Republic of Korea, the liquid waste generated from the nuclear power plants, before recycled or released into the environment, is controlled below the activity concentration limits in the effluent water through the treatment processes applying reverse osmosis, an ion exchange, and an evaporation method.
Liquid waste treatment technologies such as the reverse osmosis, the ion exchange, and the evaporation method cannot completely specify the target radionuclides to be removed from the liquid waste. Accordingly, various studies have been conducted to increase the selectivity of nuclides for the above technologies [15, 16]. On the other hand, since the aptamers can selectively remove the only specific atoms such as cobalt using the target binding characteristics, it is suggested that aptamers can be used as a removal technology of ionic radionuclides in the liquid waste.
In particular, during decommissioning the nuclear power plant, a large amount of the liquid waste is generated through the processes such as decontamination and dismantling of various structures contaminated by radioactive materials generated during the operation. Until now, adsorbents, reverse osmosis, ion exchange, and evaporation methods have been widely studied as removal technologies for radionuclides in liquid waste generated during the decommissioning of nuclear power plants, as well as after the Fukushima Daiichi nuclear accident [17–19]. By developing the aptamers with the binding capability for specific ionic radionuclides in the liquid waste, it could be applied to reducing a large amount of the liquid waste generated from decommissioning.
The aptamers consist of short oligonucleotides, a mixture of a single-strand and a double-strand as a structure analogous to hairpin structure of the single-stranded DNA (ssDNA) [20]. It is widely known that nucleic acids such as DNA can be damaged by radiation. Therefore, the radiological effect of the liquid waste on the aptamers should be evaluated quantitatively. In this paper, the radiological effect on the aptamers in the liquid waste was evaluated for the successful application of aptamers as a removal technology of the ionic radionuclides.
Materials and MethodsSo far there has been no reported evaluation of the radiological effects on the aptamers during liquid waste treatment. For this reason, we have conducted an evaluation of the radiological effect on the aptamers that can be received during the liquid waste treatment by applying a radiological damage assessment method for the DNA. DNA is damaged directly by ionizing radiation or indirectly by free radicals produced from interactions of the ionizing radiation with water, which results in strand breaks and base damages [21]. Energy deposition in the cell nucleus has been shown to be related to the radiological effect of the cells, and various studies have been conducted to apply Monte Carlo simulation methods to the assessment of DNA damage caused by the energy deposition [22].
Monte Carlo N-Particle transport code version 6 (MCNP6) [23] and Monte Carlo damage simulation (MCDS) [24–26] were used as the evaluation tools for the DNA damage in this study. MCNP6 was used to evaluate the secondary electron spectrum and the absorbed dose in a medium using photons from gamma-generating nuclides, and MCDS was used to calculate the DNA damage by using the secondary electron spectrum and the absorbed dose as inputs [27].
As the results of the MCNP6 and MCDS calculations, the damage yields of the DNA in a cell by the secondary electrons and the free radicals generated by the photons emitted from the radionuclides were derived. However, the results were not completely corresponding to the damage on the aptamers in the liquid waste, given that those results were derived for the DNA in a cell rather than for the aptamers in the liquid waste. Therefore, to apply those calculated results of the MCNP6 and MCDS for the DNA in cells to the damage on the aptamers in the liquid waste, an additional process was employed to treat and correct the differences between the DNA in a cell and the aptamers in the liquid waste. The overall evaluation process of the radiological effect on the aptamers is shown in Fig. 1.
1. Liquid Waste Generation Scenarios Applied to the Evaluation1) Scenario 1: Liquid waste generated by underwater cutting of reactor vessel internalsReactor vessel internals (RVIs) are very large components that should be segmented into small parts for the decommissioning and dismantling of nuclear power plants, but they should be generally segmented underwater remotely because they are high-radioactive components difficult for operators to access [28, 29]. Some of the radioactive materials of the RVIs’ residues after underwater cutting remain ionic in the water.
The specific activity of 60Co in a barrel among RVIs was assumed to be about 108 Bq/g based on the activation calculation results [30]. Among the radionuclides of the RVIs, the ionic radioactive materials floating in the water after the cutting were evaluated as the following. The RVIs are supposed to be cut by 1 m thickness, of which a thickness of 0.5 cm sinks into the water. At this time, if the RVIs structure is symmetric and RVIs’ weight is 100 tons, 500 kg of RVIs sinks into the water. Assuming that only 1% of RVIs’ radioactivity exists as an ionic state in water, 60Co source terms in liquid waste were derived as 500 Bq/mL when the size of the pool where the underwater cutting was performed was 10 m×10 m×10 m.
2) Scenario 2: Liquid waste generated by a wire saw cutting of bio-concreteBio-concrete surrounding the reactor core for the shielding can be radioactivated by the neutrons produced in the core. Since the bio-concrete is very bulky and thick, its radioactivity reduces as the distance from the reactor core increases [31]. Therefore, the bio-concrete should be cut for the decommissioning of a nuclear power plant according to its activity level.
A diamond wire saw is used to cut the bio-concrete, and the water is required for cooling the wire saw and for the dust control during cutting, which produces the secondary liquid waste [32]. According to the reference [31], the main gamma-ray source of the bio-concrete is 60Co and europium (Eu) isotopes (152Eu and 154Eu). The summed specific activity of the 60Co and the Eu isotopes in the bio-concrete is assumed to be about 105 Bq/g [30]. The ionic radioactive materials floating in the water by diamond wire saw cutting were evaluated by assuming as the following. The gamma energies of 1.274 MeV (35% yield) from 154Eu and 1.408 MeV (21% yield) from 152Eu are comparable to those from 60Co having two gammas of 1.17 (100% yield) and 1.33 MeV (100% yield). We employed 60Co, which is more conservative than Eu radionuclides considering both the energy and yields of europium and cobalt. In addition, by assuming that the water density is 1 g/mL and the ratio of water supplied for cutting to the cut concrete is 10 to 1, 60Co source terms in liquid waste were derived as 10,000 Bq/mL.
3) Scenario 3: Liquid waste generated by high-pressure water jetting used for surface contaminationThe high-pressure water jetting technique is effective in surface decontamination [33]. Secondary liquid waste is generated by this technique of using water for decontamination [34]. According to the radiological site characterization report of the United States [35], the maximum level of surface decontamination is about 5.03×106 Bq/cm2. In addition, since the secondary liquid waste generation rate is about 5.4 L per 1 m2 of the surface decontaminated by high-pressure water washing [34], the specific activity of about 9.32×106 Bq/mL may be contained in the liquid waste. By assuming that all surface contamination sources were 60Co, 60Co source terms in the liquid waste were derived as 9.32×106 Bq/mL. All liquid waste generation scenarios described in this section are summarized in Fig. 2.
2. DNA Damage Evaluation Using MCNP6 and MCDSMCNP6 was used to evaluate the secondary electron spectrum and the absorbed dose in the medium due to the source terms for each liquid waste generation scenario. The evaluation was conducted with an assumption that the aptamers and the 60Co sources were distributed uniformly within the 500 mL column corresponding to the size of a trial product. A cell flux tally (F4), which calculates flux averaged over a cell, of MCNP6 was used to calculate the secondary electron spectrum. Also, an energy deposition tally (F6), which calculates energy deposition averaged over a cell, was used to evaluate the absorbed dose. The photon cross-section library was applied with mcplib84 based on final release of version VI of the Evaluated Nuclear Data File (ENDF/B-VI).
MCDS was used to evaluate the DNA damage by using the secondary electron spectrum and the absorbed dose resulting from the MCNP6 calculations. MCDS results were drawn as single-strand break, double-strand break, and base damage.
3. Binding Experiments between Aptamer-Bead Complex and Ionic NuclidesBinding experiments between the aptamer-bead complex and ionic nuclides were conducted to indirectly verify the results derived by MCNP6 and MCDS calculations. Through these experiments, we have tried to estimate the possibility and extent of damage that the aptamer itself would receive by the radioactivity of nuclides by comparison assessment for amounts of removed nuclides of both stable isotope 59Co (non-radioactive) and the radioisotope 60Co by the same aptamers.
The cobalt-specific aptamer LoFA-C1 (biotin–5′–GGTAATACGACTCACTATAGGGAGATACCAGCTTATTCAATTTTGCTTGACGAGCCTGTACGTGGTTCCCTCCAGATGGTCGAGATTGCACTTACTATCT–3′) used in the experiments was selected as a specific aptamer for Co2+ by systematic evolution of ligands by exponential enrichment (SELEX) and synthesized by IDT (Integrated DNA Technologies, Inc., Coralville, IA, USA). The aptamer-bead complex was prepared by binding the aptamer to the streptavidin beads (Pierce Streptavidin Agarose; Thermo Scientific, Dreieich, Germany). Aptamer amount used in each experiment was 1.2 nmol (number of molecules: 7.2×1014).
A solution of 59Co is prepared from cobalt(II) chloride made by Sigma-Aldrich (449776-5G; Burlington, MA, USA). To measure the concentration of the 59Co in the solution after the removal of 59Co by the aptamer-bead complex, plasma-optical emission spectroscopy was used. Also, we prepared a solution of 60Co by diluting 60Co stock solution made by Eckert & Ziegler (Atlanta, GA, USA). To measure the concentration of the 60Co in the solution after the removal of 60Co by the aptamer-bead complex, the radioactivity of the 60Co was measured by the high-purity germanium detector (Mirion Technologies [Canberra] Inc., Meriden, CT, USA).
The removal rates of nuclides (59Co and 60Co) in both solutions were derived by measuring the amounts of nuclides before and after passing through the aptamer-bead complex as the relative ratios, and all experiments were repeated three times independently.
Results and Discussion1. DNA Damage in a CellTo evaluate the radiological effects on the aptamers in the liquid waste, DNA damage yields of the irradiated cells were evaluated by using MCNP6 and MCDS. The assumed 60Co radioactivity in the liquid waste was used as an initial radioactive source for the DNA damage evaluation. Table 1 shows the damage yields of DNA in a cell by the 60Co radioactivity in the liquid waste. This result shows that the damage yields of DNA in a cell are directly proportional to the assumed 60Co activity concentrations in the liquid waste streams.
2. Aptamers Damage in a CellDNA has a double-strand structure while the aptamer consists of a single-strand. This structural difference can lead to the difference in damage yields with the same radioactivity. To apply the evaluated radiological effect on the DNA in the cell calculated by MCNP6 and MCDS to the radiological effect on the aptamers in the liquid waste, a correction factor with respect to the DNA structure was used based upon the previous research [36], where the damage ratio between ssDNA and double-stranded DNA (dsDNA) was evaluated to be 3.6 with the same irradiation. Therefore, the effect magnitude by a factor of 4 was conservatively multiplied to the DNA damage yields shown in Table 1.
3. Aptamers Damage in Liquid WasteTo correct the evaluated radiological effect on the aptamers in cells to the effect on the aptamers in the liquid waste, additional study was conducted. The most abundant substance in cells is water which accounts for about 70% of the cell’s weight, but other substances such as inorganic ions and organic molecules also exist [37]. Therefore, the following evaluation was performed to consider the difference in the water content and in the chemical composition between the cell and the liquid waste.
The difference in the radiological effect due to the different chemical composition between the cell and the liquid waste was evaluated through two simple MCNP6 models. One of the models used a cytoplasm, which is the remainder of the cell except for the cell nucleus and accounts for most of the cell volume, and the other model used liquid waste as a medium. The energy deposition rates were compared between two models, one filled with liquid waste in the medium and the other filled with chemical composition of cytoplasm in the medium, with same geometry and same radioactive source terms.
F6 tally was used to calculate the energy deposition in the medium. Absorbed dose of 9.56×108 and 1.25×109 MeV/g was evaluated for the cytoplasm and the liquid waste, respectively, confirming that about 1.3 times higher energy was deposited in the liquid waste. Since DNA is damaged indirectly by the radicals generated by water radiolysis, the energy deposition in the water is important from the viewpoint of DNA damage. Accordingly, the DNA damage is assumed to be proportional to the amount of energy deposited in the water. It is also supposed that only 70% (6.69×108 MeV/g) of the energy deposited in the cytoplasm (9.56×108 MeV/g) is deposited in water because water accounts for about 70% of the cell’s weight.
Therefore, it was evaluated that the aptamers could be damaged up to twice as much in the liquid waste as in the cell according to the energy deposition in the water, and for this reason, the effect magnitude by a factor of 2 was multiplied to the DNA damage yields shown in Table 1. As a result, the damage yields of the aptamer in liquid waste were derived as shown in Table 2 by applying the effect magnitude from the structural difference between DNA and aptamer as well as the difference in the water contents between a cell and liquid waste. The aptamer damage yields were calculated per 100 base-pair (bp) since the number of bp was 100 for the template DNA used in this work.
4. Aptamers Damage per Absorbed DoseThe damage yields of aptamers for each liquid waste generation scenario can be expressed as a function of absorbed dose, and the absorbed dose evaluated for each liquid waste generation scenario is shown in Table 3. Fig. 3 shows the aptamer damage yields according to the absorbed dose, and it was confirmed that the radiological damage on aptamers increase in proportion to the absorbed dose. As a result, damage yield of about 5.00×10−4 occurs to 100 bp aptamer per radiation dose of 1 Gy. These results suggest that only very small fraction of significant number of the aptamers will be damaged by the radioactivity of the liquid waste.
5. Evaluation Results of Removal Rates of Nuclides (59Co/60Co) by the Binding ExperimentsThe above derived aptamer damages (Table 2) are resulted by the consideration of the structural difference between DNA and aptamer as well as the difference in the water contents between a cell and liquid waste. Nevertheless, there may be additional differences in the real damages on the aptamers in the liquid waste due to the factors that are difficult to quantify, such as radical scavenging effects in a cell. Since the quantitative difference caused by these additional factors is not known yet, some experiments were conducted to verify the aptamer damages indirectly. As the experiment results show, the 59Co and 60Co were removed by 48.99±2.93% and 49.22±4.68%, respectively, through the aptamer-bead complex.
These results demonstrate that the cobalt-specific aptamer LoFA-C1 could remove the two isotopes of 59Co and 60Co with very similar fraction of about 50% regardless of the presence of radioactivity. From these experimental results, it was evaluated that the damage on the binding characteristics of the aptamers by the radiation from the target nuclides such as 60Co would be negligible.
ConclusionRadiation damage of aptamers by the radioactive material in the liquid waste was evaluated in this work, so that aptamers could be used as a removal technology of radionuclides. The damage yields were evaluated to be about 5.00×10−4 for 100 bp aptamer per radiation dose of 1 Gy from the MCNP6 and MCDS calculation and application of a correction factor, which considers the structural difference between a DNA and aptamer besides the difference in the water contents between a cell and liquid waste. The evaluation results suggest that only very small fraction of a significant number of the aptamers would be damaged by the radioactive materials in the liquid waste. These analytical results were verified indirectly by the experiment that bound the stable isotope 59Co and the radioisotope 60Co to the same aptamer-bead complex, respectively, and then evaluated the nuclide removal fraction for each isotope. Furthermore, in order to practically apply the aptamers to the removal of radionuclides in the liquid waste generated from the operation and decommissioning of nuclear power plants, more study would be required to discover the factors that could damage the aptamers besides the radiological effects. Finally, it is suggested that if there are no additional damage factors except for the radiation in the liquid waste, the aptamers could be considered as a removal technology of radionuclides in the liquid waste.
AcknowledgementsThis work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20203210100390, Development of Eco-friendly Bio-material to Improve the Treatment Performance of Radioactive Liquid Waste from Decommissioning).
NotesConflict of Interest No potential conflict of interest relevant of RADCORE, Co., Ltd., to this article was reported. Ethical Statement According to Bioethics and Safety Act in the Republic of Korea, an approval from the ethics committee is not required for this study. Author Contribution Conceptualization: Yun Y, Kim SH, Kim S. Methodology: Lee M, Cha G, Kim D, Jang D, Lee S. Project administration: Kim S. Writing – original draft: Lee M. Writing – review & editing: Yun M, Kim S, Kim SH. Investigation: Lee M, Cha G, Kim D, Jang D, Lee S. Supervision: Kim S. Validation: Kim H. Approval of final manuscript: all authors. References1. Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818-822.
2. Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315-6320.
3. Wang T, Chen C, Larcher LM, Barrero RA, Veedu RN. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol Adv. 2019;37(1):28-50.
4. Ni S, Yao H, Wang L, Lu J, Jiang F, Lu A, et al. Chemical modifications of nucleic acid aptamers for therapeutic purposes. Int J Mol Sci. 2017;18(8):1683.
5. Hidding J. A therapeutic battle: antibodies vs. aptamers. aptamers [Internet]. Groningen, Nederlands, Zernike Institute for Advanced Materials, University of Groningen; 2016 [cited 2023 Feb 15]. Available from: https://www.rug.nl/research/zernike/education/topmasternanoscience/programme-documents/ns190-papers/ns_190_hidding-atherapeuticbattleantibodiesvs.aptamers.pdf
6. Zhang Y, Hong H, Cai W. Tumor-targeted drug delivery with aptamers. Curr Med Chem. 2011;18(27):4185-4194.
8. Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45(9):1628-1650.
9. Marnissi B, Kamali-Moghaddam M, Ghram A, Hmila I. Generation of ssDNA aptamers as diagnostic tool for Newcastle avian virus. PLoS One. 2020;15(8):e0237253.
10. Bauer M, Strom M, Hammond DS, Shigdar S. Anything you can do, I can do better: can aptamers replace antibodies in clinical diagnostic applications? Molecules. 2019;24(23):4377.
11. Han K, Liang Z, Zhou N. Design strategies for aptamer-based biosensors. Sensors (Basel). 2010;10(5):4541-4557.
12. Ohk SH, Koo OK, Sen T, Yamamoto CM, Bhunia AK. Antibody-aptamer functionalized fibre-optic biosensor for specific detection of Listeria monocytogenes from food. J Appl Microbiol. 2010;109(3):808-817.
13. Bouvier-Muller A, Duconge F. Application of aptamers for in vivo molecular imaging and theranostics. Adv Drug Deliv Rev. 2018;134:94-106.
14. Efremenkov VM. Radioactive waste management at nuclear power plants. IAEA Bulletin. 1989;31(4):37-42.
15. Sanchez-Marquez JA, Fuentes-Ramirez R, Cano-Rodriguez I, Gamino-Arroyo Z, Rubio-Rosas E, Kenny JM, et al. Membrane made of cellulose acetate with polyacrylic acid reinforced with carbon nanotubes and its applicability for chromium removal. Int J Polym Sci. 2015;2015:320631.
16. Shelkovnikova LA, Kargov SI, Gavlina OT, Ivanov VA, Al’tshuler GN. Selectivity of ion exchangers in extracting cesium and rubidium from alkaline solutions. Russian J Phys Chem A. 2013;87(1):125-128.
17. Awual MR, Yaita T, Miyazaki Y, Matsumura D, Shiwaku H, Taguchi T. A reliable hybrid adsorbent for efficient radioactive cesium accumulation from contaminated wastewater. Sci Rep. 2016;6:19937.
18. Lalhmunsiama L, Kim JG, Choi SS, Lee SM. Recent advances in adsorption removal of cesium from aquatic environment. Appl Chem Eng. 2018;29(2):127-137.
19. Wang J, Zhuang S. Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes. Nucl Eng Technol. 2020;52(2):328-336.
20. Jeddi I, Saiz L. Three-dimensional modeling of single stranded DNA hairpins for aptamer-based biosensors. Sci Rep. 2017;7(1):1178.
21. Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th ed. Philadelphia, PA, Lippincott Williams & Wilkins. 2012.
22. Schuemann J, McNamara AL, Warmenhoven JW, Henthorn NT, Kirkby KJ, Merchant MJ, et al. A new standard DNA damage (SDD) data format. Radiat Res. 2019;191(1):76-92.
23. Pelowitz DB. MCNP6 user’s manual version 1.0: LA-CP-13-00634 Rev. 0. Los Alamos, NM, Los Alamos National Laboratory. 2013.
24. Semenenko VA, Stewart RD. A fast Monte Carlo algorithm to simulate the spectrum of DNA damages formed by ionizing radiation. Radiat Res. 2004;161(4):451-457.
25. Semenenko VA, Stewart RD. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys Med Biol. 2006;51(7):1693-1706.
26. Stewart RD, Yu VK, Georgakilas AG, Koumenis C, Park JH, Carlson DJ. Effects of radiation quality and oxygen on clustered DNA lesions and cell death. Radiat Res. 2011;176(5):587-602.
27. Kalospyros SA, Gika V, Nikitaki Z, Kalamara A, Kyriakou I, Emfietzoglou D, et al. Monte Carlo simulation-based calculations of complex DNA damage for incidents of environmental ionizing radiation exposure. Appl Sci. 2021;11(19):8985.
28. Cooke C, Spann H. Reactor vessel and reactor vessel internals segmentation at Zion nuclear power station-13230. Conference: WM2013: Waste Management Conference: International collaboration and continuous improvement; . 2013 Feb 24–28. Phoenix, AZ. Available from: https://www.osti.gov/biblio/22224983
29. Hwang YH, Hwang S, Hong S, Park KS, Kim NK, Jung DW, et al. A study on segmentation process of the K1 reactor vessel and internals. J Nucl Fuel Cycle Waste Technol. 2019;17(4):437-445.
30. Volmert B, Bykov V, Petrovic D, Kickhofel J, Amosova N, Kim JH, et al. Illustration of Nagra’s AMAC approach to Kori-1 NPP decommissioning based on experience from its detailed application to Swiss NPPs. Nucl Eng Technol. 2021;53(5):1491-1510.
31. Evans JC, Lepel EL, Sanders RW, Wilkerson CL, Silker W, Thomas CW, et al. Long-lived activation products in reactor materials: NUREG/CR-3474 [Internet]. Richland, WA, Pacific Northwest Lab; 1984 [cited 2023 Jan 10]. Available from: https://doi.org/10.2172/6776358
32. International Atomic Energy Agency. Decontamination and demolition of concrete and metal structures during the decommissioning of nuclear facilities. IAEA Technical report series No 286. Vienna, Austria, International Atomic Energy Agency. 1988.
33. Remark JF. A review of plant decontamination methods: EPRI—NP-6169 [Internet]. Palo Alto, CA, Electric Power Research Institute; 1988 [cited 2023 Jan 10]. Available from: https://doi.org/10.2172/6625352
34. Larsson H, Anunti A, Edelborg M. Decommissioning study of Oskarshamn NPP [Internet]. Stockholm, Sweden, Svensk Kambranslehantering AB; 2013 [cited 2023 Jan 10]. Available from: https://www.skb.com/publication/2625263
35. U.S. Nuclear Regulation Commission. Trojan nuclear plant: radiological site characterization report: ML003670710 [Internet]. Washington, DC, NRC; 1995 [cited 2023 Jan 10]. Available from: https://www.nrc.gov/docs/ML0036/ML003670710.pdf
36. Piedade JA, Oliveira PS, Lopes MC, Oliveira-Brett AM. Voltammetric determination of gamma radiation-induced DNA damage. Anal Biochem. 2006;355(1):39-49.
37. Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, et al. Essential cell biology. 4th ed. New York, NY, Garland Science. 2014.
Fig. 1The evaluation process of radiological effect on the aptamers. MCNP6, Monte Carlo N-Particle transport code version 6; MCDS, Monte Carlo damage simulation. 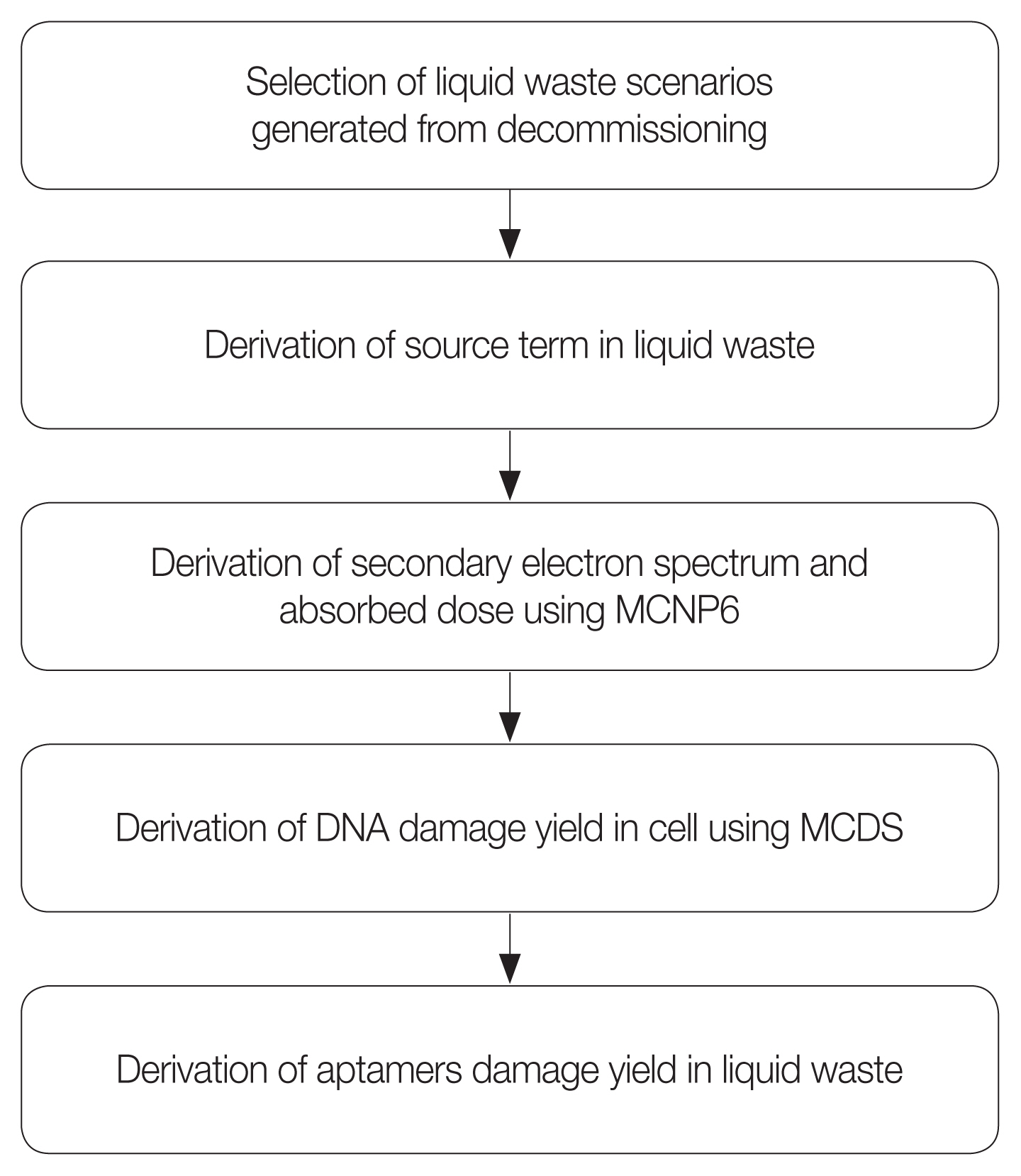
Table 1Damage Yields of DNA in a Cell by 60Co Radioactivity from Liquid Waste Generation Scenarios Table 2Damage Yields of the Aptamer (Based on 100 bp) in the Liquid Waste by 60Co Radioactivity from Liquid Waste Generation Scenarios Table 3Absorbed Dose by 60Co Radioactivity from Liquid Waste Generation Scenarios |
|
|||||||||||||||||||||||||||||||||||||||||||