AbstractBackgroundAs new legislation has come into force implementing radiation safety management for the use of naturally occurring radioactive materials (NORM), it is necessary to establish a rapid and accurate measurement technique. Measurement of 238U and 232Th using conventional methods encounter the most significant difficulties for pretreatment (e.g., purification, speciation, and dilution/enrichment) or require time-consuming processes. Therefore, in this study, the applicability of ED-XRF as a non-destructive and rapid screening method was validated for raw materials and by-product samples.
Materials and MethodsA series of experiments was conducted to test the applicability for rapid screening of XRF measurement to determine activity of 238U and 232Th based on certified reference materials (e.g., soil, rock, phosphorus rock, bauxite, zircon, and coal ash) and NORM samples commercially used in Korea. Statistical methods were used to compare the analytical results of ED-XRF to those of certified values of certified reference materials (CRM) and inductively coupled plasma mass spectrometry (ICP-MS).
Results and DiscussionResults of the XRF measurement for 238U and 232Th showed under 20% relative error and standard deviation. The results of the U-test were statistically significant except for the case of U in coal fly ash samples. In addition, analytical results of 238U and 232Th in the raw material and by-product samples using XRF and the analytical results of those using ICP-MS (R2≥0.95) were consistent with each other. Thus, the analytical results rapidly derived using ED-XRF were fairly reliable.
IntroductionPublic interest in radiation has grown since the Fukushima nuclear accident has prompted a greater need to manage naturally occurring radioactive material (NORM), which exists alongside artificial radionuclides. According to the International Commission on Radiological Protection publication 103 (ICRP-103), NORM is defined as material containing naturally occurring radionuclides—including cosmic rays—that were generated with the creation of the Earth [1], and its danger could increase due to radiation exposure as the material is concentrated through artificial manufacturing processes by human activities. Due to such concerns, the Natural Radiation Safety Management Act was enforced in 2012, and in terms of radiation safety management, raw materials and by-products that simultaneously exceed a certain quantity and concentration level (235U, 238U, 232Th, and their daughters: 1 Bq·g−1, 40K: 10 Bq·g−1) must be registered with an agency and regulated in order to manage radiation exposure for workers and consumers who produce and use raw materials and by-products. In addition, the Act specifies the standards of raw materials (40K: 1 Bq·g−1, 235U, 238U, 232Th, and their daughters: 0.1 Bq·g−1) and by-products (40K: 5 Bq·g−1, 235U, 238U, 232Th, and their daughters: 0.5 Bq·g−1) in terms of radiation (Act on Protective Action Guidelines Against Radiation in the Natural Environment, Legislation 12664, May 21, 2014).
The radioactivity analysis of naturally occurring radionuclides can be divided into two methods: the direct measurement method, in which the concentration of the mother nuclide (235U, 238U, 232Th) is directly measured, and the indirect measurement method, in which a secular equilibrium between the mother nuclide and daughter nuclide is hypothesized. Some direct measurement methods include alpha spectrometry, liquid scintillation counting (LSC), ICP-MS, and gamma-ray spectrometry, which is used to directly measure 40K and is also used as an indirect measuring method in which the daughter nuclide is analyzed to infer the radioactivity of the mother nuclides 238U and 232Th [2–4]. The advantage of alpha spectrometry and LSC is that the radioactivity concentration of naturally occurring radionuclides can be directly analyzed, but some disadvantages include the prolonging of the total duration of analysis since the medium must be completely decomposed, a complicated chemical pre-treatment (e.g. a purification process in which interfering substances and nuclides within the samples are removed) that must accompany the analysis, and the increase of the uncertainty factor during the pretreatment process [5–7]. Sample pretreament is relatively simple for gamma-ray spectrometry, but approximately 30 days are needed for radioactive equilibrium and a long measurement time (usually around 24 hours) is needed as well. However, an inaccurate value may be derived due to radioactive disequilibrium that may occur in most by-product or raw material samples that undergo chemical treatment processes, depending on the chemical characteristics of the elements [8–10].
Meanwhile, unlike direct measurement methods of radation such as alpha spectrometry, liquid scintillation counting (LSC), and gamma-ray spectrometry, mass measurement methods such as ICP-MS, energy dispersive X-ray fluorescence spectrometry (ED-XRF), thermal ionization mass spectrometry (TIMS), laser induced breakdown spectrometry (LIBS), particle induced X-ray emission (PIXE), neutron activation analysis (NAA), and delayed neutron activation analysis (DNAA) can also be used to measure the radioactivity concentration of nuclides. The calculation of a mass-radioactivity relationship (specific activity), which uses the decay time of the nuclide and the atomic weight is presented in Equation 1. The specific activity for 235U, 238U, and 232Th are 12.5, 80.34, and 46.0 μg·Bq−1, respectively, and the final concentration of the nuclide is determined by the abundance ratio of the isotope. Therefore, application of a mass spectrometric method is more effective than a radiometric measurement method for nuclides that have a long half-life, such as 238U and 232Th, and application of a radiometric method is more effective than a mass spectrometric method for nuclides that have a short half-life of less than 100 years, such as 55Fe and 63Ni, since the specific activity for such nuclides is very high [11, 12].
Mass spectrometric analysis can be divided into the destructive method, which completely decomposes the samples, and the non-destructive method, which preserves the samples. Destructive methods such as ICP-MS, atomic absorption spectrometry (AAS), TIMS, and LIBS show excellent results in terms of analytic sensitivity and accuracy, but since they require sample pretreatment, the rapidity of analysis and on-site applicability are relatively weak. Meanwhile, in terms of analytical accuracy, non-destructive methods such as PIXE, NAA, and DNAA show excellent results, but since they require nuclear reactors and accelerators, accessibility is low. On the other hand, ED-XRF is a non-destructive method that measures the concentration of the elements by analyzing the characteristic X-rays that are emitted from in the sample after excitating the sample using X-rays, and its dominant advantages are simple sample preparation compared to other measurement methods and simultaneous multielement analysis [13–15].
There are relatively few case studies that use ED-XRF for the analysis of naturally occurring radionuclide concentration. Trojek et al. proposed the applicability of the ED-XRF method on the detection of uranium and thorium, and D′ Cunha et al. used wave length-dispersive XRF (WD-XRF) in order to assess the concentration of 232Th for mineral samples containing monazite. Meanwhile, Johnson conducted XRF and alpha spectrometry for a U analysis of the soil of a polluted site, and proposed the application of ED-XRF for concentrations below 90 ppm [16–18].
Rapid screening is essential in efficiently regulating and managing the analysis of naturally occurring radionuclides in raw material and by-product samples by reducing the time and cost of analysis. In choosing the appropriate analysis method for screening, the versatility and speed of the equipment, convenience, and analytical accuracy are important assessment factors. Among these, ED-XRF could meet factors like the universality of the equipment, rapidity of analysis, and convenience.
Therefore, this study assessed the applicability of ED-XRF as a rapid screening method regarding the radioactivity concentration of 238U and 232Th among raw material and by-product samples by analyzing certified reference materials (e.g., soil, rock, phosphate rock, bauxite, zircon, and coal ash) and assessed the credibility of the analytical results of uranium and thorium concentration. In addition, this study used ED-XRF to analyze raw material samples (e.g. zircon, bauxite) and by-product samples (e.g. coal ash) that are actually distributed in South Korea and compared the results with ICP-MS analytical results.
Materials and MethodsIn order to validate the ED-XRF analytical results, certified reference materials similar in medium to the targeted raw material and by-product samples were selected. The certified reference materials used in this study were 2709a (San Joaquin soil), 1633c (trace elements in coal fly ash), 1646a (estuarine sediment), 278 (obsidian rock), and 694 (western phosphate rock) of the U.S. National Institute of Standards and Technology (NIST), BX-N (bauxite) of the National Center for Scientific Research (CNRS), and 388 (zircon) of the Bureau of Analyzed Samples Ltd (BCS). Table 1 presents the actual raw material and by-product samples that are distributed in South Korea, including 18 types of raw materials and 9 types of by-products: vermiculite, bentonite, clay, zircon, bauxite, ceramic ball, coal fly/bottom ash, and zircon dust. For atypical or coarse particle samples such as vermiculite, ceramic ball, zircon, and bauxite, a disk grinding machine (8530 Enclosed Shatterbox, SPEX SamplePrep, Metuchen, NJ) was used to homogenize the particle sizes of the samples. Since samples subject to grinding mostly have a high level of specific gravity, grinding was impossible with the commonly used agate, so they were grinded with a disk and container made of tungsten carbide. The average particle diameter of the ground particles was approximately 3 μm, and afterwards, the particles were dried for about 3 hours at 100°C. The ground and dried particles were then transferred into a cylindrical plastic sample cup (ф 32 mm) about 1 cm in height with mylar film attached for the ED-XRF analysis.
1. Analysis equipmentThe ED-XRF (Xepos HE, Spectro, Kleve, Germany) in this study uses a tungsten anode X-ray generator with a maximum tube voltage of 50 kV, and can selectively quantify the concentration of the elements using 8 secondary targets. The detector used was a silicon drift detector type of a peltier cooling module and the peak resolution is 155 eV at Mn-Kα. The X-ray path is in two perpendicular planes and the scatter noise from the generator tubes was minimized using polarization. The fluorescent x-rays were measured using cartesian geometry among the secondary targets, samples, and the detector. For the ED-XRF measurement, the geochemistry trace powder method provided by the manufacturer for environmental/mineral sample measurement was applied, and the ED-XRF measurement was repeated 7 times each for a total of 30 minutes using secondary targets made up of cobalt, molybdenum, aluminum oxide, and zinc. The correction of the mass attenuation effect on the sample medium, a modified algorithm for peak overlap, and a revised algorithm for Compton peak sizes were applied to the method used for this analysis. Table 2 presents analytical conditions, such as the secondary targets used according to target elements, the tube voltage (kV) and electrical current (mA) for X-ray tubes, and measurement times. When analyzing most samples—including raw material and by-product samples—using ED-XRF, the matrix effect produced depending on the components, background spectrum, elemental content, and peak overlap that could arise depending on the composition could be considered as uncertainty factors in the results.
In order to minimize these inteferences, the geochemical method employed in this study used a fundamental parameter method that applied basic physical parameters to modify the background spectrum by repeatedly recalculating the spectral peaks that occurred due to various electronic transitions of an element initially obtained from the spectrum. In addition, the geochemical method reproduced spectrum by modifying errors caused by peak overlap and absorption edge. In order to modify the changes in matrix and equipment stabilization, an algorithm was applied that modifies the coefficient of the total spectrum with regard to the intensity of Compton peaks. Moreover, to offset the matrix effect, the ratio of Compton scattering and Rayleigh scattering was analyzed for standard samples of approximately 25 species to calculate and apply the mass attenuation coefficient. Regarding the influence of peak overlap for characteristic energy, rubidium and strontium were quantified for uranium, while iron, rubidium, and strontirum were quantified for thorium for each characteristic peak. Peak separation was conducted by subtracting the corresponding area from the uranium peak. Such X-ray acquisition, correction, and analysis were automatically conducted using the X-LapPro program. The calibration curve of uranium, thorium, and component elements were prepared using various types of standard materials, such as soil and rock samples. For uranium and thorium, the determination coefficient (R2) of the calibration curve from sub-ppm to % of concentration range was 0.9981 and 0.9946, respectively. As shown in Figure 1A, the concentrations of uranium and thorium were analyzed using the molybdenum secondary target and the Lα characteristic X-ray of 13.612 keV and 12.967 keV, respectively. Meanwhile, during metallic element analysis using ED-XRF, the analytical result (or concentration level) was greatly influenced by adjacent peaks. As shown in Figure 1B, for uranium (Lα, 13.612 keV), if the concentration of rubidium is relatively high due to peak overlap caused by the adjacent rubidium (Kα, 13.390 keV) line, the analytical results could be overestimated or the detection limit could be higher.
Results and Discussion1. Validation of ED-XRF determinationFor the analysis of trace elements in the raw material and by-product samples that used ED-XRF—especially that of uranium and thorium—method validation for the entire analysis process including sample preparation, estimation, and result management is an important provision for determining the reliability of the analytical results. The accuracy (or relative error) is determined by the difference of the measurement for the certified value of the certified reference material. The relative standard deviation (RSD, standard deviation/mean of experimental value, %) of the measurements denotes the precision of the analytical values. Both are used as criteria to determine the validity of the analysis method and process. In addition, U-test (an index that evaluates the trueness of the analytical value regarding the ceritifed value through statistical methods other than relative error and RSD) results are specified in Table 3. The results were calculated by dividing the difference between the analytical value and the certified value and the sum of the uncertainty of the certified value and the measured value. Systematic bias of the measured values did not appear in the 95% or 99% confidence levels if the U-score did not exceed 1.95 or 2.58, and the results could be considered statistically reliable [19, 20].
In this study, ED-XRF was used to analyze uranium and thorium in various certified reference materials from various raw material mediums (e.g. soil, rock, coal ash, bauxite, and zircon) and by-product mediums. The analysis was conducted in accordance with the conditions outlined in Table 2. The results of the analysis are organized in Table 3. According to Table 3, all analytical results showed a relative error of less than 10%, except for uranium (37.6%) in the coal ash medium SRM 1633c and thorium (18.6%) in the medium CNRS Bx-N. Relative standard deviation, which shows analytical precision, in the all results showed less than 10%, except for cases in which the concentrations of uranium and thorium were less than 10 mg·kg−1.
Moreover, as presented in Table 3, accuracy evaluation for all analytical results shows that values were statistically reliable, as U-test results were all less than 1.96, except for the results of the SRM 1633c and SRM 694 uranium analysis. For SRM 1633c (4.4), the U-test result exceeded 2.0 and the relative error was relatively high at 37.6%. These results reflect the influence of peak overlap caused by the rubidium within the medium. However, based on the result that the confidence interval of the relative standard deviation of the experimental values was low, the modifying algorithm for the peak overlap must be supplemented. In addition, the U experimental value for SRM 694 showed a 2.0 U-test result, but this result is due to the extremely low relative expanded uncertainty of the certified value of the standard material (approximately 0.425%). Even in this case, however, although the relative error of the analytical results in this study was approximately 10.3%, the relative standard deviation was 2.28%. Thus, one may declare the analytical results of the phosphate rock medium is considered reliable with very precise measurement.
Figure 2 presents the ED-XRF measurements of uranium and thorium regarding the certified reference materials. A linear regression analysis was conducted in order to identify the variability of the results according to the range of concentration levels. As a result, the regression coefficient—the deviation of the two variables regarding the mean—was 0.9914, and the variation coefficient—the dispersion of the deviation—was 0.9945, which shows a great linearity in a wide range of concentration from 3.0 to 350 mg·kg−1. When the concentrations of 238U and 232Th were converted into mass concentrations in terms of the registration standards of the raw materials or by-products, the values were 80.4 mg·kg−1 and 246 mg·kg−1. The mass concentrations that correspond to the raw material standard of 0.1 Bq·g−1 were 8.04 mg·kg−1 and 24.6 mg·kg−1, which magnified in detail the concentration range of raw material standards outlined in Figure 2. As shown in Figure 2, a linear relationship was also maintained in the low concentration range of below 30 mg·kg−1.
2. Comparison of the methods for the 238U and 232Th analysisThe applicability of an analysis using ED-XRF is very good as a rapid screening method since it can rapidly measure the checmical composition of samples and at the same time, can directly infer the radioactivity concentrations of 238U and 232Th, the mother nuclides of naturally occurring radionuclides. Therefore, ED-XRF is considered the most effective analytical method in terms of on-site applicability and convenience since it can be applied to monitor the concentration levels of naturally occurring radionuclides for samples of the same medium during the processing phase and on site. However, because the ED-XRF analysis is highly dependent on the sample medium, the establishement of fundamental parameters (e.g. correction of the mass attenuation effect on the sample matrix, modifying algorithms for peak overlap, etc.) is essential in order to improve the accuracy of the analytical results [21–23]. Therefore, in order to identify the uncertainty of these mediums, the ED-XRF analytical results were intercompared to the ICP-MS analytical results of the raw material samples (e.g. zircon, bauxite) and by-product samples (e.g. coal ash) actually distributed in Korea. The ICP-MS analytical results were cited from references that verified the development and validity of the analytical method [24, 25].
Measurements using ED-XRF and ICP-MS were conducted 3 times. The natural isotopic abundance ratios (238U: 99.3%, 232Th: 100%) were applied to convert into the radioactivity concentration. The radioactivity concentration of 238U and 232Th in the 27 types of raw material and by-product samples analyzed using ED-XRF were presented in Table 1 and Figure 3. Based on the results, the mean radioactivity concentration of 238U and 232Th were 1.074 Bq·g−1 (0.028–4.283 Bq·g−1) and 0.471 Bq·g−1 (0.005–4.408 Bq·g−1), respectively. 238U showed the highest mean value of 2.370 Bq·g−1 in the zircon matrix of the raw materials, and 75% of the samples were assessed to be over the registration standard of 1.0 Bq·g−1, which was similar to the concentration range (238U: 1–4 Bq·g−1, 232Th: 0.5–1 Bq·g−1) found in world-wide commercial zircon in the previous study [26]. The 238U concentration of the zircon dust sample was also found at a high level of 0.892 Bq·g−1 (0.354–1.604 Bq·g−1). In the case of bauxite and ceramic ball, the concentration level was approximately 0.5 Bq·g−1, while bentonite and coal ash showed a low concentration level of less than 0.1 Bq·g−1. The concentration of 232Th was 4.408 Bq·g−1 for the ceramic ball sample, which was about 4.4 times higher than the registration standard, and this sample was analyzed to be part of the alumina series with the following element distribution: SiO2 64.5%, Al2O3 19%, Fe2O3 7.2%, TiO2 0.8%. The radioactivity concentrations of 232Th appeared to be relatively high, similar to those of 238U in raw materials such as zircon and bauxite, but when compared to 238U in terms of radioactivity concentration, it was distributed in relatively low concentration levels.
In order to evaluate the applicability of ED-XRF in the radioactivity analysis of 238U and 232Th in raw materials and by-products, the analytical results of ED-XRF and ICP-MS were intercompared and presented in Figure 4. To be clear the analytical results of ICP-MS were set as the standard and the relative difference percent (RDP) between the methods was calculated and schematized. As shown in Figure 4, there was a high correlation between the analytical results from the two methods. The regression coefficients for 238U and 232Th were 0.916 and 1.055, respectively, and the variation coefficients were 0.954 and 0.992, respectively. However, the concentrations of 238U and 232Th show a relatively higher difference for a few zircon matrix samples and zircon process dust samples. As shown in Figure 4A, the 8 sets of data that deviated from the linear drift curve were all samples made up of zircon components. As shown by the RDP analytical results in Figure 4B, the concentration difference between the two screening methods for a certain medium appears frequently in the area of radioactivity concentration below 0.5 Bq·g−1. It is considered that such analytical uncertainty was caused by the mass attenuation effect for sample mediums in the ED-XRF analysis. Therefore, in order to analyze the raw material and by-product samples of the zircon series, additionally building fundamental parameters is required. Moreover, it is considered that the concentration difference of more than 20% as shown in Figure 4 for vermiculite and coal ash samples is caused by the relative increase in measurement uncertainty in terms of the detection limit level (0.1- a few mg·kg−1) of the ED-XRF equipment. Therefore, nevertheless it is difficult to quantify low levels of 238U and 232Th during analysis using ED-XRF, the method is considered excellent in terms of analytical accuracy and convenience for application as a rapid screening method for radioactivity concentration levels of more than 0.5 Bq·g−1 regarding various medium samples.
ConclusionThis study assessed the applicability of ED-XRF as a rapid screening method regarding naturally occurring radionuclides (238U and 232Th) among raw material and by-product samples. For the validation of the ED-XRF measurement, the 8 certified standard materials similar in medium to the targeted raw material and by-product samples were used. The analyzed values showed good accuracy and precision within ±20% of the certified value. Furthermore, the analyzed values were statistically significant, which indicates that the applicability of ED-XRF is excellent. Finally, when compared to the analyzed values of ICP-MS regarding actual raw material and by-product samples, the concentration difference between the two methods was not significant in the area of concentration levels greater than 0.5 Bq·g−1. Therefore, as a non-destructive rapid screening method that does not require chemical pretreatment of samples, the ED-XRF method can accurately analyze radioactivity for 238U and 232Th concentrations of raw material greater than registration standards, and can be used as a rapid screening method on site or within the laboratory.
AcknowledgementsThis research was financially supported by the Korea Institute of Nuclear Safety under the project “Establishment of Technical Basis for Implementation on Safety Management for radiation in the Natural Environment”
References1. International Commission on Radiological Protection. The 2007 recommendations of the international commission on radiological protection. ICRP Publication 103. 2007;27.
2. Xhixha G, et al. The worldwide NORM production and a fully automated gamma-ray spectrometer for their characterization. Radioanal Nucl Chem. 2013;295:445-457.
3. Mas JL, Villa M, Hurtado H, Garcia-Tenorio R. Determination of trace element concentrations and stable lead, uranium and thorium isotope ratios by quadruple-ICP-MS in NORM and NORM-polluted sample leachates. J Hazard Mater. 2012;205–206:198-207.
4. Baik MH, Kang MJ, Cho YS, Jeong JT. A comparative study for the determination of uranium and uranium isotopes in granitic groundwater. J Radioanal Nucl Chem. 2015;304:9-14.
5. L’Annunziata MF. Handbook of radioactivity analysis. 3rd ed. New York, NY, Academic Press. 2012;364-416.
6. Valkovic V. Radioactivity in environment. 1st ed. New York, NY, Elsevier. 2000;199-204.
7. Garner J, Cairns J, Read DJ. NORM in the east midlands’ oil and gas producing region of the UK. Environ Radioact. 2015;150:49-56.
8. International Commission on Radiological Protection. Radiation protection and NORM residue management in the titanium dioxide and related industries. Safety Report Series No.76. 2012;53.
9. Papadopoulos A, Christofides G, Koroneos A, Stoulos S, Papastefanou C. Radioactive secular equilibrium in 238U and 232Th series in granitoids from Greece. Appl Radiat Isot. 2013;75:95-104.
10. Ji YY, Chung KH, Lim JM, Kim CJ, Jang M, Kang MJ, Park ST. Analytical evaluation of natural radionuclides and their radioactive equilibrium in raw materials and by-products. Appl Radiat Isot. 2015;97:1-7.
11. Hou X, Roos P. Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal Chim Acta. 2008;608:105-139.
12. Becker JS. Mass spectrometry of long-lived radionuclides. Spectrochim Acta Part B. 2003;58:1757-1784.
13. Verma HR. Atomic and nuclear analytical methods. 1st Ed. New York, NY, Springer. 2006;1-2.
14. Imanishi Y, BandoAKomatani S, Wada S. Experimental parameters for XRF analysis of soils. Powder Diffr. 2010;248-255.
15. Boyle JF. Rapid elemental analysis of sediment samples by isotope source XRF. J Paleolimnol. 2000;3:213-221.
16. Trojek T, Čechák T. Detection of terrestrial radionuclides with X-ray fluorescence analysis. Radiat Prot Dosimetry. 2015;164:529-532.
17. D’Cunha P, Narayana . Comparison of methodologies of gamma ray spectrometer and x-ray fluorescence. Indian J Pure Appl Phys. 2012;50:524-526.
18. Johnson RL, Durham LA, Rieman GR, Hummel JE. Triad case study: Rattlesnake Creek. Remediation. 2004;15:69-77.
19. International Atomic Energy Agency. IAEA analytical quality in nuclear applications. Series No.32. 2011;12-15.
20. Thompson M, Wood R. The international harmonized protocol for the proficiency testing of (chemical) analytical laboratories. Pure Appl Chem. 1993;65:2123-2144.
21. Feret FR, Hamouche H, Boissonneault Y. Spectral interference in X-ray fluorescence analysis of common materials. Powder Diffr. 2003;46:381-387.
22. Espen PV, Lemberge P. ED-XRF spectrum evaluation and quantitative analyzing multivariate and nonlinear techniques. Powder Diffr. 2000;43:560-569.
23. Ramsey MH, Potts PJ, Webb PC, Watkins P, Watson JS, Cloes BJ. An objective assessment of analytical method precision comparison of ICP-AES and XRF for the analysis of silicate rock. Chem Geol. 1995;124:1-19.
24. Korea Atomic Energy Research Institute. Development of methods for the determination of 235, 238U, 226Ra, 232Th and 40K in Raw materials or by-products. KAERI-CR-529. 2013;98-101.
25. Korea Atomic Energy Research Institute. Development of analytical procedures and method validation for quantification of natural radionuclides in raw materials or by-products. KAERI-CR-576. 2015;63-65.
26. International Atomic Energy Agency. Radiation protection and NORM residue management in the zircon and zirconia industries. Safety Report series No.51. 2007;119.
Fig. 1(A) XRF spectrum with Molybdenum target and (B) spectral overlapping of Uranium and Rubidium. 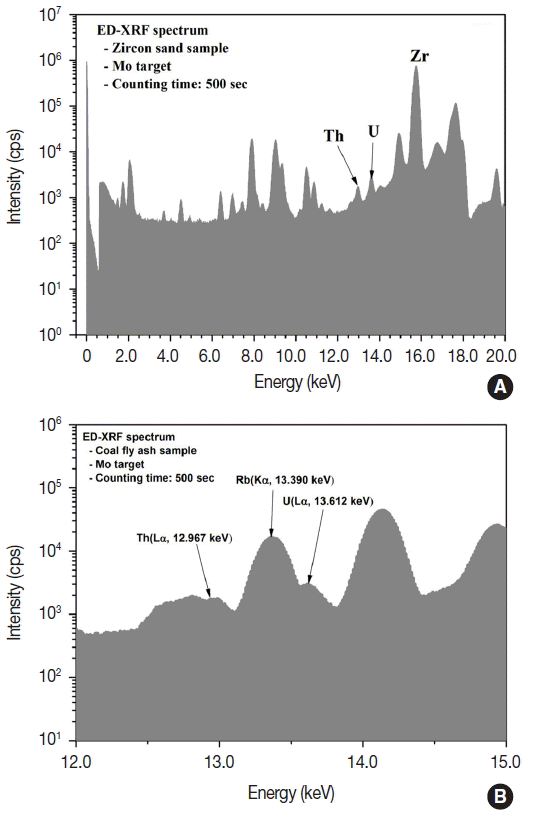
Fig. 4(A) Comparison of results between XRF and ICP-MS determination and (B) relative difference percent (RD) based on the concentrations from ICP-MS. 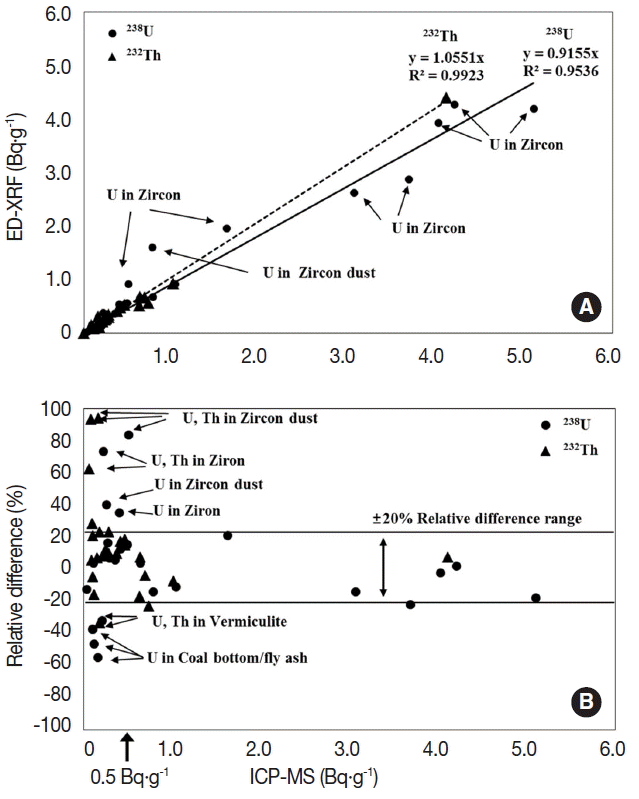
Table 1Sample Description for NORM Analysis and Activity Ranges from XRF Results (Bq·g−1)
Table 2Analytical Conditions of XRF Determination Table 3Accuracy and Precision of ED-XRF Measurement with Various Certified Reference Materials
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||