Changes of Activity Concentration of Tritium in Water under the Air with Tritiated Water Vapor
Article information
Recently, controversy continues over the cause of occurrence of tritiated water, whose activity concentration was about 7.1×108 Bq·m−3, found in a nuclear power plant in Korea. The power plant claims that the tritiated water may have been produced by the absorption of the tritiated water vapor from the air, while some suspect that it may have leaked out from the other systems [1]. We cannot hastily determine the exact cause but we have investigated the possibility that water containing initially no tritium could be changed to tritiated water with high activity concentration of tritium when it is placed in the air containing tritiated water vapor.
It has been well known since the 1960s that tritium in the air containing tritiated vapor (HTO, T is tritium or 3H) is easily transferred in water. This phenomenon has been used early for sampling of tritium in the air (known as air bubbler) [2–4] and still used today. It is also applied to respiratory masks for removing tritiated water vapor these days [5–7].
The relationship between the molar concentrations of tritium and hydrogen in the tritiated vapor of the air and those in (liquid phase) water at equilibrium is expressed by Equation (1), reported by Sepall and Mason [8], as follows:
where T is tritium, H is hydrogen, and α is the partition constant (or equilibrium constant) and is 0.91 at 20°C.
In the above equation, molar concentrations of tritium in vapor and water can be converted to the activity concentrations of tritium in vapor and water, respectively, by the following equation:
where m is the number of moles of tritium in vapor or water, V is the volume of vapor or water, A is the radioactivity of tritium in vapor or water, λ is the decay constant of tritium, 6.023×1023 is Avogadro’s number, and S is the activity concentration of tritium in vapor or water.
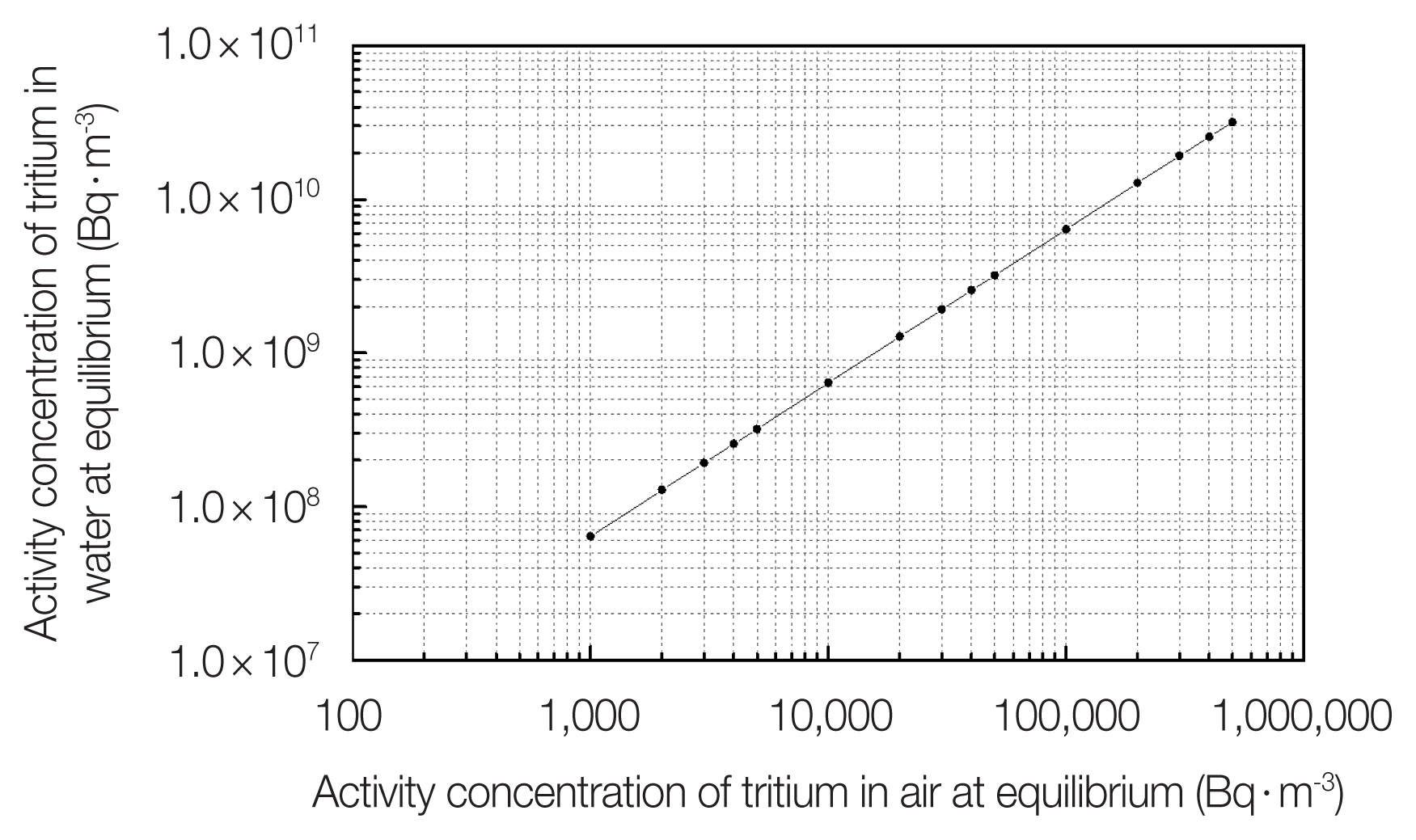
The activity concentration of tritium in the air and that in water at equilibrium are calculated by using above two equations and the result is shown in Fig. 1.
An air with activity concentration of 3,000 Bq·m−3 (release limit of tritium in air with tritiated water vapor in Korea [9]) equilibrates at 20°C with a water with activity concentration of 1.92×108 Bq·m−3. Also, at the same temperature, an air with activity concentration of 300,000 Bq·m−3 (derived concentration of tritium in air with tritiated water vapor in Korea [9]) equilibrates with a water with activity concentration of 1.92×1010 Bq·m−3.
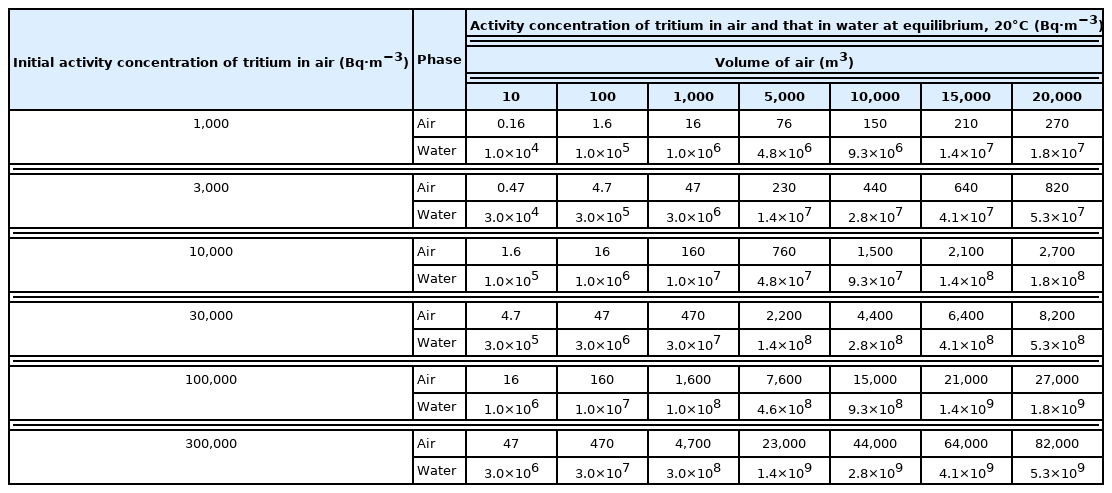
When a 1 m3 of water with no tritium is placed in certain volume of air with 50% RH (relative humidity) at 20°C and tritiated vapor, the activity concentration of tritium in air and that in water at equilibrium after saturation by the water and tritiated water vapors are shown in Table 1.
When a 1 m3 of water with no tritium is placed in a volume of air with 50% RH at 20°C, containing tritium, the activity concentration of tritium in air and that in water at equilibrium are changed with the volume of air. When volumes of air with initial activity concentration of 3,000 Bq·m−3 are 10 m3, 100 m3, 1,000 m3, 5,000 m3, 10,000 m3, 15,000 m3, and 20,000 m3, respectively, the activity concentrations of tritium in air at equilibrium are reduced to 0.47 Bq·m−3, 4.7 Bq·m−3, 47 Bq·m−3, 230 Bq·m−3, 440 Bq·m−3, 640 Bq·m−3, and 820 Bq·m−3, respectively, and those in water increase to 3.0×104 Bq·m−3, 3.0×105 Bq·m−3, 3.0×106 Bq·m−3, 1.5×107 Bq·m−3, 2.8×107 Bq·m−3, 4.1×107 Bq·m−3, and 5.3×107 Bq·m−3, respectively. It indicates that the activity concentration of tritium in water placed in the air of 3,000 Bq·m−3 and 15,000 m3 could exceed the release limit of tritiated water in Korea (4×107 Bq·m−3) [9]. If the initial activity concentration of tritium in air is 300,000 Bq·m−3, the equilibrium activity concentration of tritium in water placed in the air of above 150 m3 exceeds the release limit of tritiated water.
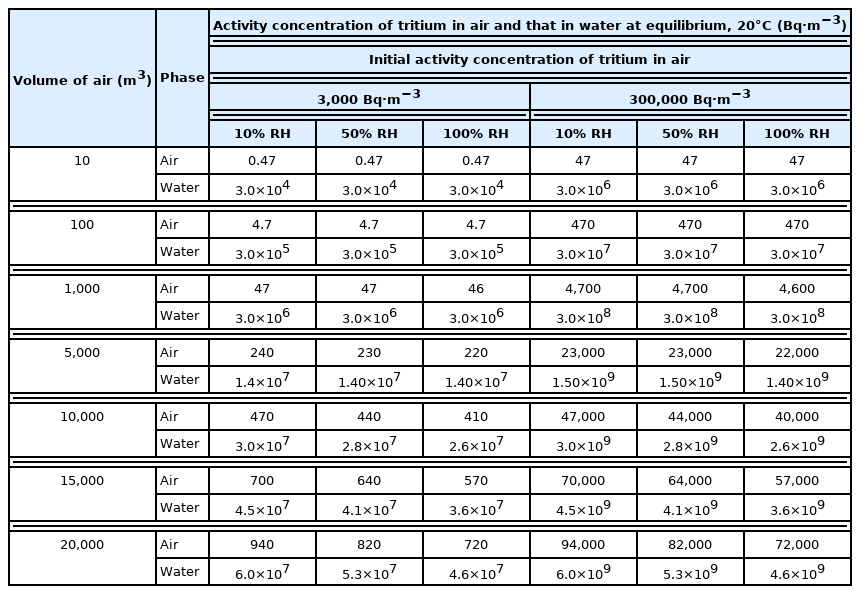
Changes of the activity concentration of tritium in air and that in water at equilibrium with relative humidity at 20°C are shown in Table 2.
The lower the initial relative humidity of air, although the activity concentration of tritium is the same, the higher the activity concentration of tritium in air and that in water at equilibrium. The reason is that the lower the relative humidity, the more evaporation of water until it reaches the equilibrium and the volume of water decreases.
As described above, we briefly estimates the change of the activity concentration of tritium in water when the water is placed in the air containing tritiated water vapor. We have confirmed that water with initially no tritium could be changed to tritiated water with high activity concentration. At present, we are planning to conduct experiments to verify that this phenomenon occurs in the field.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contribution
Methodology: SP Yim, CK Lee. Investigation: SP Yim, CK Lee. Writing - original draft: SP Yim. Writing - review & editing: CK Lee.